
The Electric Battery
A brief overview and explanation of the chemical and electric reaction within a battery.
The Electric Battery
The electric battery or cell produces power by means of a chemical reaction, although there are exceptions such as the nuclear battery. A battery can be either primary or secondary: the primary type is normally regarded as un-chargeable whereas a secondary cell or storage battery can be recharged.
There are some indications that batteries may have been used by the Parthians, a tribe in what is now Iran, for electroplating jewellery in the third century BC, but the work which led to modern batteries began with the discovery, in the early nineteenth century by Italian, Alessandro Volta, that he could cause an electric current to pass through a wire by immersing two different metals in a salt solution.
All batteries be it primary or secondary types, work as a result of a chemical reaction. This reaction produces an electric current because the atoms of which chemical Elements (pure substances) are made, are held together by electrical forces when they react to form compounds.
The outer layer of an atom is composed of Electrons, tiny particles each carrying a negative electrical charge. These particles are not all permanently attached to their atoms. In all but a few elements (the rare gases), there are invariably loosely bound electrons that can migrate between atoms during chemical reactions.
When an atom gains an electron, it gains an extra negative charge, and so becomes negatively charged as a whole. When it loses one, on the other hand it becomes positively charged. Atoms or groups of atoms in this charged state are known as IONS. Positive and negative ions are attracted to each other, and when circumstances allow, will move together and combine to form COMPOUNDS. Ions with similar charges repel each other.
The electrons of the atoms of a metal are very easily detached (therefore metal conducts electricity better than other substances). An electric current consists of a flow of electrons through a metal, hopping from atom to atom.
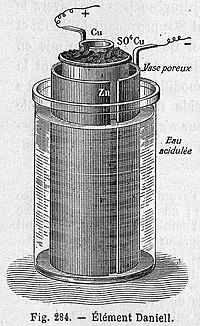
A simple cell consists of two conductors, plates of metal or carbon, dipped into a water-based solution, the electrolyte. Pure water cannot be used because it is a very good electrical INSULATOR and would block the flow of current, but when certain chemical substances are dissolved in it, the water becomes a conductor. Suitable chemical substances fall into three categories. There are the acids such as sulphuric acid; bases or alkalis such as caustic soda; and salts formed by the interaction of an acid and a base.
Electricity is generated in cells because when any of these chemical substances is dissolved on water its MOLECULES break up and become electrically charged ions. a good example is sulphuric acid, H2SO4, the molecules of which consists of two atoms of hydrogen, one of sulphur and four oxygen. When dissolved in water the molecules split into three parts; the two atoms of hydrogen separate and in this process each loses and electron, becoming a positively charged hydrogen ion (represented by the sign H+). The sulphur atom and four atoms of oxygen remain together as a sulphate group (SO4) and acquire the two electrons lost by the hydrogen atoms, thus becoming negatively charged (written SO4--). These groups can combine with others of opposite charge to form other compounds.
If one plate or electrode of zinc and one of their copper or carbon is dipped in sulphuric acid electrolyte and each is externally connected to a load such as a lightbulb, a current will flow through the bulb, lighting it. This is because of one of the chemical elements chosen for the electrodes is electrically positive (that is, has a tendency to lose electrons and acquire a positive charge) with respect to the other, and when they are electrically connected the chemical equilibrium of the cell is upset and reactions start at both plates. Under these circumstances, the atoms of zinc each give up two electrons which flow through the external circuit and form the current. The positively charged zinc atoms left behind dissolve into the electrolyte and each one combines with one of the negatively charged sulphate ions. The result is a neutral zinc sulphate molecule. The two electrons originally given up by each zinc atom travel around the external circuit and reach the other plate. There they combine with and neutralize the positive charges on two hydrogen atoms from the electrolyte. These two hydrogen atoms then combine to form a molecule of hydrogen gas, and gas bubbles are produced at the plate or electrode.
In theory, the chemical reaction would go on and electric current would continue to flow until all the zinc on the zinc plate (known as the negative electrode or cathode) has been used up. But in the simple cell a film f hydrogen bubbles begins to form on the copper or carbon plate (known as the positive electrode or anode), and as hydrogen has a much higher electrical resistance than the electrolyte proper, the internal resistance of the cell increases reducing the current that can flow in the external circuit. At the same time, a voltage (a difference in electrical charge) is produced between the hydrogen and the zinc, and this voltage is in opposition to the main voltage produced between the zinc and the copper or carbon plate, further reducing the available voltage and current.
If the external circuit is disconnected, the hydrogen bubbles will gradually disappear and the cell can be used again, but the same thing will re-occur. In all modern batteries this effect, which is known as polarization, is greatly reduced by surrounding the positive electrode with material known as depolarizer. This works either by reacting with the hydrogen to form water or be taking over from the hydrogen task of accepting the electrons as they arrive from the external circuit.
The Leclanché cell
The “dry” batteries used in flashlights and so on, which have depolarizer, are of a type known as Leclanché with modifications to make the liquid electrolyte a semisolid. In its original form the Leclanché cell was entirely “wet” with electrolyte consisting of a strong solution of ammonium chloride. A zinc plate was used for the negative electrode, and carbon rod packed into a porous pot containing crushed carbon and manganese dioxide (to accept the electrons) formed from the positive electrode and its depolarizer. Similar materials are used in a modern dry Leclanché cell. The electrolyte is not, in fact, dry but is made up in the form of a moist paste or jelly.
Additives include mercuric chloride introduced to inhibit what is known as local action. This is the name given to the chemical reactions that occur between zinc atoms and carbon and iron atoms which occur as impurities in the zinc plate. It can be overcome by a process known as amalgamation in which the mercury forms an amalgam or alloy with the zinc. – an effect that would reduce the shelf life of the battery, that is, the length of time it can be stored without deterioration.
In the cylindrical Leclanche cell used in flashlights the zinc forms the outer casing and is also the negative electrode. The positive electrode consists of a mixture of graphite (carbon) and manganese depolarizer around a graphite rod.
Dry cells are supplied singly or in groups of two, three or more to give higher voltages. This is known as a series connection. And the positive electrode of one cell is connected to the negative electrode of the next. High voltage batteries, in which sixty or more individual cells are connected in series, are available but are very heavy and cumbersome. A lighter and more compact construction, where large number of cells are to be connected in series, is the flat or layer type. Batteries of this type consists of alternate thin, flat layers of zinc electrolyte and the materials making up the positive electrode and its respective depolarizer.
Other batteries
A disadvantage of Leclanche cells is that the current quickly falls, principally because hydrogen forms more quickly than it can be removed by the depolarizer. For this reason, they are best suited to intermitted work. A more constant voltage and current is provided by the mercury cell widely used in hearing aids where almost continuous operation is necessary. In this type of cell, the full name of which is zinc-mercuric oxide, the electrolyte consists of potassium hydroxide. The negative electrode is zinc, as in the Leclanche cell, but the positive electrode and its depolarizer consists of graphite and mercuric oxide.
A lead-acid battery, as used in a car, does not practice consist simply of two plates dipped in acid. Lead dioxide is too brittle, and while pure lead is rigid, the lead that is deposited on the negative plate by the charging process builds up as a sort of metal sponge. Which is very fragile. Consequently, both plates need some sort of support.
This is normally accomplished by making the plates in the form of grids of an alloy of lead and antimony (which is tougher than pure lead) with the spongy lead or lead dioxide pressed into the grid. There are several grids in each cell of the battery arranged so that positive and negative alternate. They are spaced a short distance apart by separators to make the construction more rigid.
A single cell, that is, one set of plates immersed in a single container of acid. Produces electricity at the comparatively low voltage of 2V. the electrical system of most modern automobiles runs on 12v; this is provided by linking six cells together in series (end-to-end) so as to take advantage of their combined voltage. The six cells are completely separate except for linking bars across the top to carry the current from one to another.
A lead-acid battery must not be allowed to remain discharged for a long time. This is because the lead sulphate, which is deposited in microcrystalline form (in small crystals), tends to harden into a solid block. Once it has done this it will not take part in any chemical reaction and the plate becomes partly or wholly dead, depending on how much of it is affected. This change is known as sulphating.
The state of charge of a battery can easily be discovered by measuring the density the acid. As the battery discharges so the acid turns to water and the lighter it becomes. Its density is measured wit a hydrometer. When the battery is recharged, it becomes warm. Some of the water evaporates, so storage batteries must be topped up occasionally with pure, distilled water.
Other types
The lead-acid battery is a simple and durable device but is rather heavy. Other, lighter types of battery have been invented. The most important of these re the NiFe (pronounced knife) cell invented by Thomas Edison, and the Nickel Cadmium cell.
The NiFe cell is light and robust, but each cell of a battery only produces 1.2V instead of 2V of a lead-acid cell. As a result, more cells are needed to produce a given voltage. This, and greater manufacturing cost, make the NiFe system considerably more expensive. It is used in portable radio transmitters because of its lightness. Nickel Cadmium cells are becoming lighter and more powerful, as well as cheaper, and are being developed for use in high-performance electric vehicles.
The main area of research has been to replace the conventional lead-acid battery with a smaller, more efficient unit. This would greatly increase the practicality of electric vehicles, especially for city use where range and speed are less critical factors.
One possibility is the sodium-sulphur battery which could, in theory, achieve an energy density of 2.5MJ per kg. The sodium-sulphur battery is unusual because it is made from B-alumina which is a form of aluminium oxide. This allows sodium ions to pass freely through at a high temperature, usually around 570°F (300°C). At this temperature the sodium and sulphur are both liquid and are held apart by a solid alumina tube which constitutes the electrolyte. The cells are packed into thermally insulated stainless steel canister which controls the heat loss. At the present state of development, it is possible to get an energy density for such batteries better than 0.5MJ per kg.

